Reliably at the service of Greek pharmacies

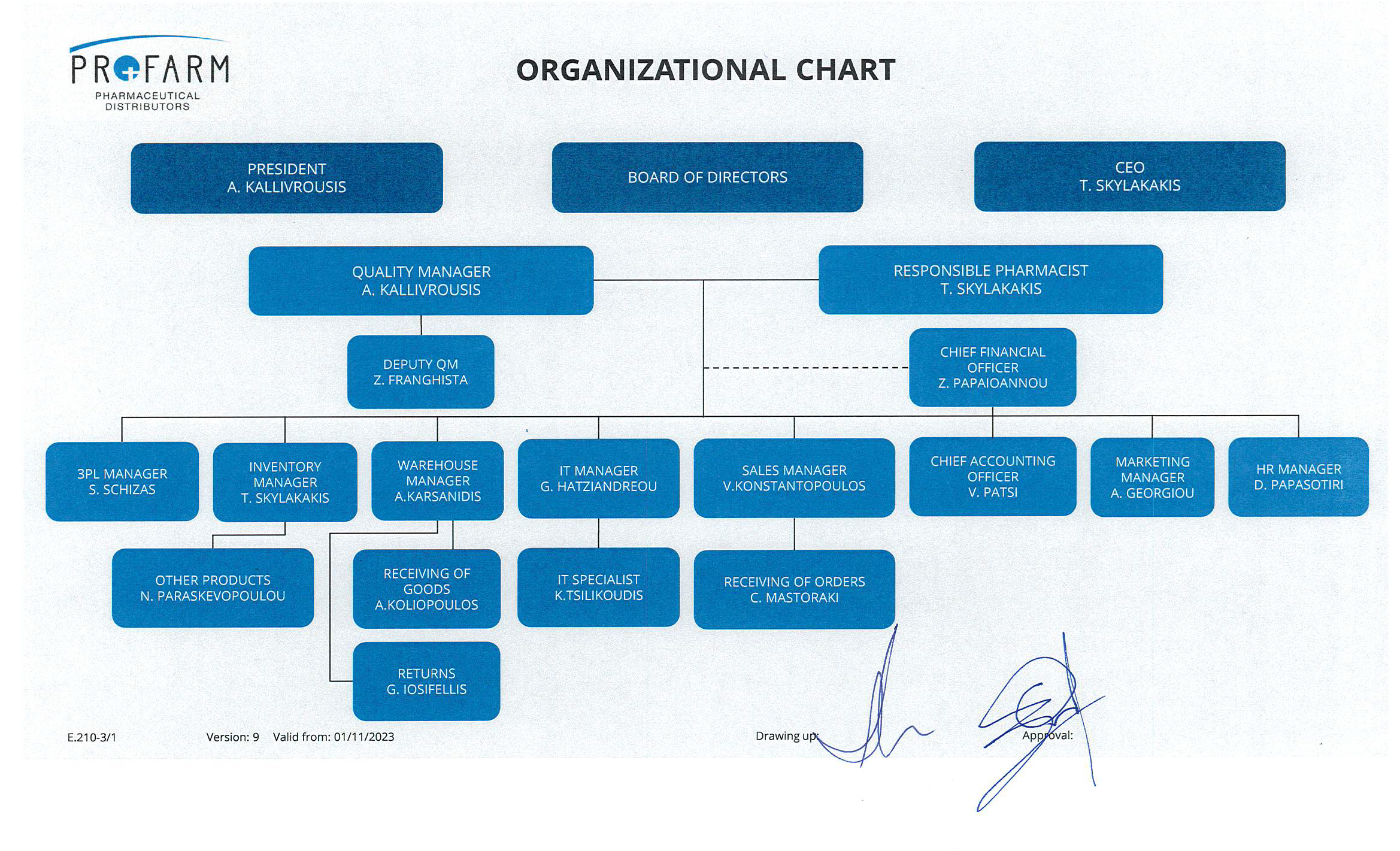
The Profarm Group was founded in 1993, initially called D. Stasinopoulos & Co. O.E., by four pharmacists, Messrs. A. Kallivroussis, Th. Skylakakis, G. Kamenakis and D. Stasinopoulos with a paid-up capital of 6,000,000 drachmas. In 1995 it was renamed Th. Skylakakis and Co. O.E. following the departure of D. Stasinopoulos, and in 2004 assumed its current legal form: PROFARM S.A.
With a 30-year experience in pharmaceutical trading, it supplies more than 1000 pharmacies and 130 pharmaceutical distributors in Greece (via the third-party logistics division) and is one of the leading companies in the pharmaceuticals trading field.
In addition, it owns 29% of Pharmanet S.A. (pharmaceutical distributor in Agrinio) and 13% of Stargen Ltd. (pharmaceutical company active in generics).
In 2018 absorbs pharmaceutical distributor V. Tsitsas and strengthens its third-party distribution services and the provision of Logistic management and storeroom organisation services.
In addition, in 2019 Profarm acquires 50% of Medworld, a company active in the exclusive import and marketing of parapharmaceutical products, offering high-tech products to the Greek pharmacy. In 2020, Emma Capital enters the share capital of Profarm Group with the aim of further growth. A new cycle of mergers and acquisitions begins. At the same time, the commercial pharmaceutical company Per Se Pharmaceuticals S.A. is founded. In 2021, the companies El-Pharm S.A. and Intergis Pharma Ltd (holder of marketing authorisations for orphan drugs), join the Profarm group.
Since 2006, Profarm S.A. has adopted the Quality Principles and has maintained its certification according to International Standard ISO9001 in its occasionally updated version by an Independent Certification Provider.
Since 2013, by decision of EOF/Health Ministry, Profarm S.A. has a permit to attach a certification band ensuring authenticity for pharmaceutical products.
In May 2018, with sensitivity and a feeling of social and environmental responsibility, Profarm S.A. developed and is practicing a system of environmental administration according to International Standard ISO 14001:2015 and was certified by an Independent Certification Provider.
In June 2018 Profarm S.A. received certificate of Approval by an Independent Certification Provider according to the demands included in paper no. ΔΥ8δ/1348ΓΠ/οικ.1348:2004 (ΦΕΚ 32 Β/16-1-2004) of the Greek Ministry of Health and Welfare for the movement of medical-technical products.
Profarm Group today holds a leading position in the field of distribution of pharmaceutical and para-pharmaceutical products. With the immediate and quality service provision to pharmacies their target, Profarm Group constantly develops their distribution network with their modern fleet of temperature-controlled vans, which covers not only pharmacies’ daily needs but also any urgent order.
The products are delivered safely and reliably by professional drivers using company-owned distribution vehicles that fulfil the specification for safe transport and delivery of pharmaceutical and para-pharmaceutical products (Good Distribution Practices).
Profarm Group, whose primary target is to respond to the needs of pharmacies, ensures daily sufficiency of pharmaceutical and para-pharmaceutical products.
The Board of Profarm, with securing their clients’ satisfaction as their guiding principle and having as their top priority the health of the consumers, is committed to applying a Unified System for Quality Control per EN ISO 9001-2015, EN ISO 14001-2015 as well as the occasional rules for Correct Storage and Distribution (GSP’s and GDP’S).
The application of the Unified System for Quality Control fully reflects the operation of the company, following all relevant Greek and EU legal requirements, aiming at continuously improving our effectiveness while respecting all involved - clients, employees, the environment and all others involved in whatever way.
Within this framework, targets and effectiveness ratings are set and constantly monitored in order to ensure:
- The understanding of the needs of current and potential customers and the adaptation of our targeting to match their desires.
- The constant training of our personnel and the recognition of our people in the chain of value added and continuous improvement of the operation of our business.
- The provision of up-to-date equipment and health & safety conditions in the workplace for our people.
- The development of relationships of trust with an extended network of rated associates and suppliers through sound practices and exchanges of knowledge.
- The use of up-to-date technological methods and equipment for the provision of high-quality distribution services of suitable and safe products.
- Prevention of pollution with the constant reduction of environmental impact caused by our operations.
- Constant improvement of our company processes through the management of risks but also opportunities, as determined through observing the market and its developments.
- In cooperation with Red Cross, we organise days for voluntary blood donation for enrolment of new volunteer bone marrow transplant donors for employees of Profarm Group and all associated pharmacies. By helping voluntary blood donation, we help those people who are in need.
- We support the work of the S.G.F.CS.G.F.CS.G.F.C organization (SAVE GREECE FROM CANCER).
- We also support, through participating and by donating, the charitable race No Finish Line. In this race the kilometres are converted into money with the aim of supporting the work of the charitable organisation Together for Children so they can fulfil their program of feeding needy families with underage children.